MY EVIDENCE.
MY CHOICE.
MYOBLOC.


Backed by clinical studies, MYOBLOC® is the first and only FDA-approved botulinum toxin B for the treatment of chronic sialorrhea in adults. See why physicians consider MYOBLOC for their patients.
Jump to Section
collapseSialorrhea can be a challenging symptom of Parkinson’s disease (PD)
Affecting up to 80% of patients with PD, sialorrhea can be both underrecognized and undertreated.1,2
Sialorrhea can cause life-threatening complications.*,3-5
Aspiration pneumonia carries ~20% mortality in patients with PD.6-8
Sialorrhea can affect patients by causing3,5,9-11:
Speech disturbances
Sleep disruption
Social stigma
Caregiver burden
Depression
Skin infection
*There are no studies showing that treating sialorrhea affects specific outcomes on this page.
“I’ll be reading...next thing I know, I’ve got drool all over the place. You’ll get a napkin or paper towel to wipe it off...in 15 minutes it may be the same thing again.”
George,† living with PD
“It was bad, it was really bad. I could follow him in the house…he was drooling all the way to wherever he was going. He didn’t even realize it.”
Margaret,† PD caregiver
Are your patients with PD aware that chronic sialorrhea is a separate and treatable condition?
†Actual patient and caregiver testimonials.
MYOBLOC has a distinct mechanism of action12

Stage 1: MYOBLOC binds to receptors on the neuronal surface via the heavy chain.
Stage 2: MYOBLOC enters the nerve cell via endocytosis and is contained in vesicles.
Stage 3: MYOBLOC light chain moves to the cytosol where it cleaves synaptic vesicle-associated membrane protein (VAMP)* essential for acetylcholine (ACh) release.
*VAMP is a component of the protein complex responsible for docking and fusion of the synaptic vesicle to the presynaptic membrane, a necessary step in neurotransmitter release.
For chronic sialorrhea, blocking ACh release from the neurosecretory cells of the salivary glands reduces saliva production.12

Proven clinical efficacy and tolerability for chronic sialorrhea
SELECT A DATA SET
-
A phase 3, multicenter, randomized, double-blind, placebo-controlled, single-treatment efficacy and safety study
A phase 3, multicenter, randomized, double-blind, placebo-controlled, single-treatment efficacy and safety study
- 187 adult patients enrolled
- Objectives: Determine efficacy, safety, and tolerability over a 13-week period
- Unstimulated Salivary Flow Rate (uSFR) and Clinical Global Impression of Change* (CGI-C) at Week 4: Primary endpoints
- Patient Global Impression of Severity (PGI-S), Patient Global Impression of Change (PGI-C), Drooling Impact Score (DIS), Drooling Frequency and Severity Scale (DFSS): Secondary endpoints
-
A phase 3, multicenter, randomized, double-blind, placebo-controlled, single-treatment efficacy and safety study
A phase 3, multicenter, randomized, double-blind, placebo-controlled, single-treatment efficacy and safety study
- 187 adult patients enrolled
- Objectives: Determine efficacy, safety, and tolerability over a 13-week period
- Unstimulated Salivary Flow Rate (uSFR) and Clinical Global Impression of Change* (CGI-C) at Week 4: Primary endpoints
- Patient Global Impression of Severity (PGI-S), Patient Global Impression of Change (PGI-C), Drooling Impact Score (DIS), Drooling Frequency and Severity Scale (DFSS): Secondary endpoints
-
A phase 3, multicenter, randomized, double-blind, placebo-controlled, single-treatment efficacy and safety study
A phase 3, multicenter, randomized, double-blind, placebo-controlled, single-treatment efficacy and safety study
- 187 adult patients enrolled
- Objectives: Determine efficacy, safety, and tolerability over a 13-week period
- Unstimulated Salivary Flow Rate (uSFR) and Clinical Global Impression of Change* (CGI-C) at Week 4: Primary endpoints
- Patient Global Impression of Severity (PGI-S), Patient Global Impression of Change (PGI-C), Drooling Impact Score (DIS), Drooling Frequency and Severity Scale (DFSS): Secondary endpoints
-
A phase 2, multicenter, double-blind, placebo-controlled, sequential dose-escalation study
A phase 2, multicenter, double-blind, placebo-controlled, sequential dose-escalation study
- 54 adult patients enrolled
- 4 dose arms: 1,500 Units (n=14); 2,500 Units (n=12); or 3,500 Units (n=13) vs. matching placebo (n=15)
- Patients were followed for up to 20 weeks after injection
- Primary outcome measures: Safety and tolerability
- All efficacy measures were considered secondary
- uSFR and CGI-C were measured at Week 4 and other selected time points
Significant improvement in uSFR was demonstrated at Week 4 for all doses tested11-13
At Week 4, MYOBLOC 2,500 U and 3,500 U achieved:
Early, significant, and enduring effect11-13
MYOBLOC treatment resulted in a significantly greater reduction in uSFR vs. placebo
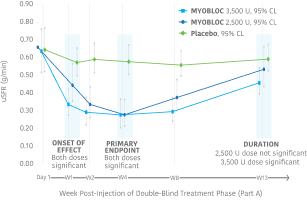
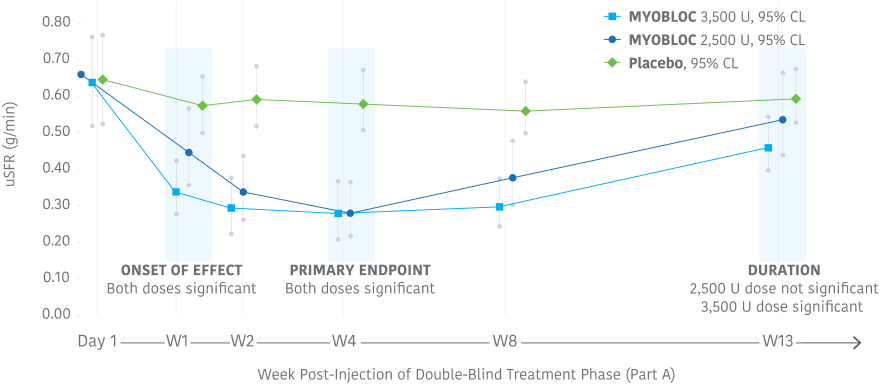
- Reductions began at Week 1 and continued through Week 8 for both doses tested
- Significant effect continued through Week 13 for the 3,500 Unit dose
In the Phase 3, open-label extension (Study 1, Part B), improvement in uSFR was maintained for over 1 year with subsequent dosing of MYOBLOC 3,500 U.†
†Limitations associated with open-label study design, include lack of comparator arm, decreasing sample size and potential continued involvement of responders and attrition of nonresponders.
Significant improvement in CGI-C was demonstrated at Week 4 for all doses tested11-13
At Week 4, MYOBLOC 2,500 U and 3,500 U achieved:
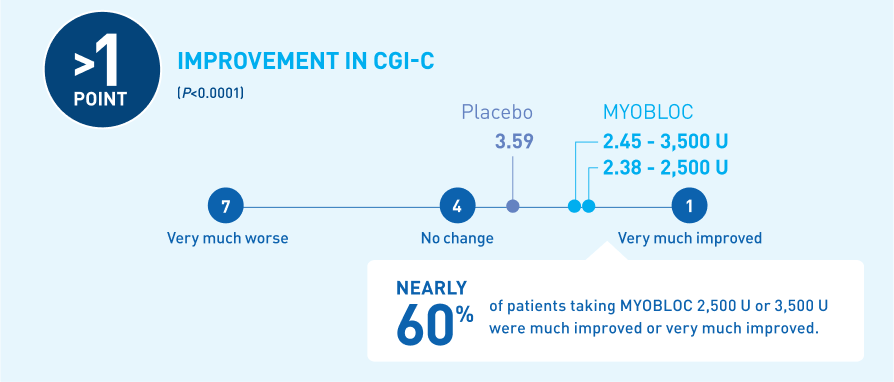
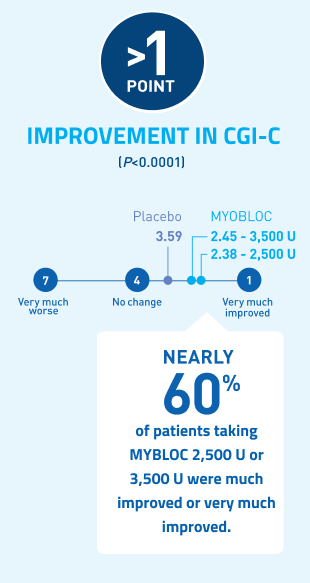
*Chronic sialorrhea was "much improved" or "very much improved" according to CGI-C score at Week 4 post-injection in patients treated with MYOBLOC 2,500 Units (60%) and 3,500 Units (53%) compared to patients on placebo (12%).
In the Phase 3, open-label extension (Study 1, Part B), improvement in CGI-C was maintained for over 1 year with subsequent dosing of MYOBLOC 3,500 U.†
†Limitations associated with open-label study design, include lack of comparator arm, decreasing sample size and potential continued involvement of responders and attrition of nonresponders.
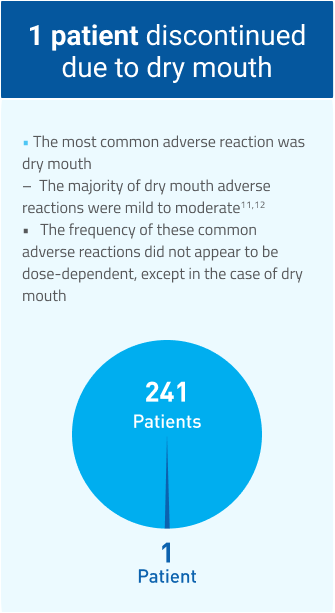
MYOBLOC has demonstrated tolerability11-13
Studies 1 and 2
Adverse reactions in at least 5% of MYOBLOC-treated patients and greater than placebo in pooled chronic sialorrhea studies
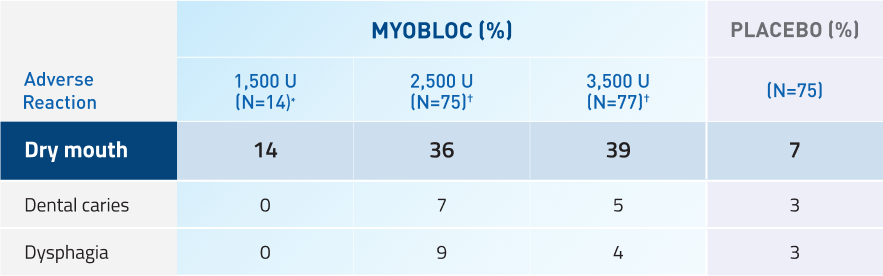
*Adverse reactions for 1,500 Unit dose were only evaluated in Study 2.
†Adverse reactions for 2,500 Unit and 3.500 Unit doses were evaluated in Studies 1 and 2.

Take the next step with MYOBLOC
-

Order MYOBLOC
Order MYOBLOCOrder by phone or email and we'll help you get the treatment your patients need.
-

Get Live Support
Request A SpecialistGet in touch with a Reimbursement Specialist who can help you navigate patient support services, insurance benefits, and more.
-

Access Resources
View ResourcesFind resources that can help guide you through the process of injecting MYOBLOC.
