What patient types are
right for MYOBLOC?


Whether you are a frequent injector or a new injector of MYOBLOC®, see possible patient types who could be appropriate to treat with MYOBLOC.
-

Jim, 74, Has Cervical Dystonia
Meet Jim, who was diagnosed with cervical dystonia 10 years ago. Jim is no longer feeling relief from his botulinum toxin-A injections as his neck is tightening more lately, affecting his mobility.
 Download Profile
Download Profile
-

Lydia, 44, Has Cervical Dystonia
Meet Lydia, a patient with cervical dystonia. Over the past 4 years, she has experienced gradually worsening twisting of her neck with pain, with limited relief from physical intervention and oral medication.
 Download Profile
Download Profile
-

Ryan, 39, Has Cervical Dystonia
Meet Ryan, a patient who has experienced 6 months of pain and tightness in his neck due to cervical dystonia. Even after a year of physical therapy, he’s been unable to find relief.
 Download Profile
Download Profile
-

Gwen, 71, Has Cervical Dystonia
Meet Gwen, an active grandmother who has been experiencing persistent neck pain and tightness. Her primary care physician referred her to a physical therapist with the goal to find relief.
 Download Profile
Download Profile
-

Donna, 68, Has Chronic Sialorrhea
Meet Donna, a patient recently experiencing chronic sialorrhea from Parkinson’s disease. Her drooling is beginning to affect her life and social interactions.
 Download Profile
Download Profile
-

Charles, 72, Has Chronic Sialorrhea
Meet Charles, a patient with advanced Parkinson’s disease and diagnosed with chronic sialorrhea 2 years ago. His chronic sialorrhea affects his speech to loved ones.
 Download Profile
Download Profile
For cervical dystonia in adults
Dosing and administration
recommendations for
cervical dystonia1
For cervical dystonia in adults

Whether you need a refresher on dosing ranges or want to gain a better understanding of the injection process, here are some resources to guide you every step of the way.
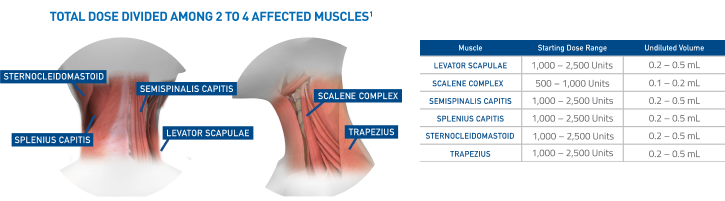
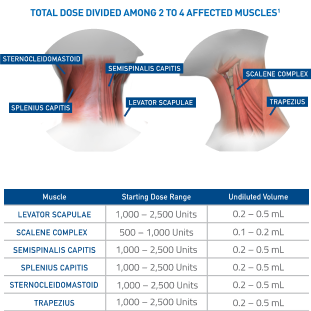
Initial dosing recommendations for affected muscles1
Recommended initial total dose to be divided among affected muscles:
- Patients with a prior history of tolerating botulinum toxin injections is 2,500 to 5,000 Units
- Patients without a prior history of tolerating botulinum toxin injections should receive a lower initial dose
- Subsequent dosing should be optimized according to the patient’s response


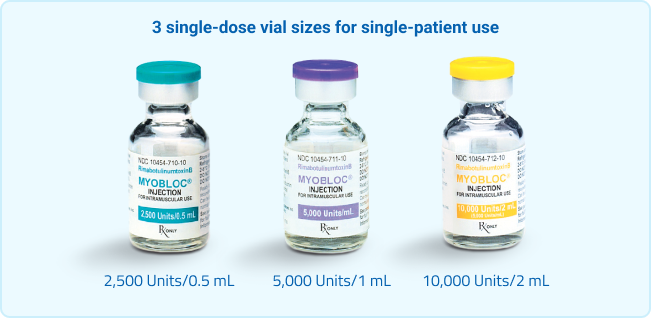
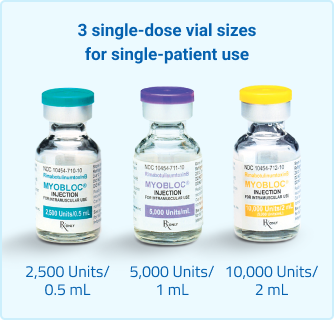
Proven, versatile, and requires no mixing1
With no reconstitution required, MYOBLOC® is syringe-ready and available in 3 vial sizes for single-patient use. For injection, you'll need a suitable sterile needle (eg, 30-gauge, 0.5 inch) and alcohol swabs.
IMPORTANT SAFETY INFORMATION WARNINGS AND PRECAUTIONS
Lack of Interchangeability Between Botulinum Toxin Products
The potency units of MYOBLOC are specific to the preparation and biological activity assay method utilized. Due to differences in the aspects of this assay such as the vehicle, dilution scheme, and laboratory protocols for various potency assays, potency units are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of MYOBLOC cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method.
Download these MYOBLOC guides for your office
Cervical Dystonia Injection Guide
This guide offers detailed instructions for injecting MYOBLOC for the treatment of cervical dystonia.
Cervical Dystonia Anatomical Guide
This guide helps you keep a record of where your patient’s injections should be administered and the dosage strength used for the treatment of cervical dystonia.
No Mixing Guide
This guide provides dosing instructions, recommendations, and considerations for the only botulinum toxin that doesn’t require mixing, MYOBLOC.
Order MYOBLOC
If you’re ready to get started with MYOBLOC, you may choose below to see ordering information or start by requesting samples.


For Chronic Sialorrhea in adults
Dosing and administration
recommendations for
chronic sialorrhea1
For Chronic Sialorrhea in adults

Whether you need a refresher on dosing ranges or want to gain a better understanding of the injection process, here are some resources to guide you every step of the way.
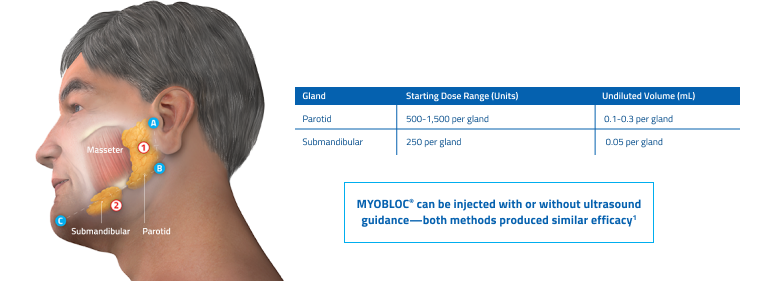
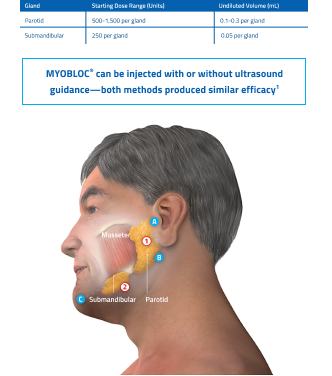
Guidelines for anatomical landmarks1
- Parotid gland injection
- Bisect the distance between the tip of the tragus (Site A) and the angle of the mandible (Site B)
- Inject 1 finger breadth anterior to this site (Injection Site 1)
- Submandibular gland injection
- Bisect the distance between the angle of the mandible (Site B) and the tip of the chin (Site C)
- Inject 1 finger breadth medial to the inferior surface of the point of bisection (Injection Site 2)




Proven, versatile, and requires no mixing1
With no reconstitution required, MYOBLOC is syringe-ready and available in 3 vial sizes for single-patient use. For injection, you'll need a suitable sterile needle (eg, 30-gauge, 0.5 inch) and alcohol swabs.
IMPORTANT SAFETY INFORMATION WARNINGS AND PRECAUTIONS
Lack of Interchangeability Between Botulinum Toxin Products
The potency units of MYOBLOC are specific to the preparation and biological activity assay method utilized. Due to differences in the aspects of this assay such as the vehicle, dilution scheme, and laboratory protocols for various potency assays, potency units are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of MYOBLOC cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method.
Dosing recommendation1
- Subsequent dosing and frequency of dosing should be optimized according to the patient’s individual response
- Generally, no more frequent than every 12 weeks
- Individual patient responses may vary
Dosing considerations1
- Sialorrhea symptom severity
- Risk of dysphagia—patients with neuromuscular disorders should be monitored closely for severe swallowing or breathing difficulty
Download these MYOBLOC guides for your office
Chronic Sialorrhea Injection Guide
This guide offers detailed instructions for injecting MYOBLOC for the treatment of chronic sialorrhea.
Chronic Sialorrhea Anatomical Guide
This guide helps you keep a record of where your patient’s injections should be administered and the dosage strength used for the treatment of chronic sialorrhea.
No Mixing Guide
This guide provides dosing instructions, recommendations, and considerations for the only botulinum toxin that doesn’t require mixing, MYOBLOC.
Order MYOBLOC
If you’re ready to get started with MYOBLOC, you may choose below to see ordering information or start by requesting samples.


Access and
coverage resources


Find information on our co-pay program as well as our patient assistance program to help your patients access and save on MYOBLOC®.


Co-Pay Brochure
This brochure helps you and your staff better understand the MYOBLOC co-pay program, including how to enroll patients, benefits, and eligibility requirements.


Co-Pay Assistance
For more information about our Patient Programs, visit the patient financial assistance page, see FAQs below, or call 1-888-461-2255, Option 3.
Monday through Friday, 8 AM-8 PM ET.
MYOBLOC Financial Assistance Programs
For eligible patients who need financial assistance in obtaining MYOBLOC treatment, we offer the following patient programs:
MYOBLOC Co-Pay Program
-
The MYOBLOC Co-pay Program assists eligible patients with cervical dystonia with their out-of-pocket expenses associated with MYOBLOC and the related administration expenses. With no limit per injection, eligible patients may receive up to $4,000 per year of assistance with permitted out-of-pocket expenses.1
-
Patients who are 18 years or older, diagnosed with cervical dystonia (G24.3) or chronic sialorrhea (K11.7), a legal US resident, who have commercial insurance coverage according to the terms and conditions of the program and are NOT enrolled in a government insurance plan (eg, Medicare, Medicaid, TRICARE®, and other federal- or state-funded programs).2
-
No enrollment forms needed. HCP staff can enroll patients by calling 1-888-461-2255, Option 3. Patients can enroll themselves by calling 1-888-461-2255, Option 3, as well.
-
Once eligible for participation, the patient’s eligible out-of-pocket expenses may be paid directly to the site of care (administering office or pharmacy) on the patient’s behalf, or to the patient as a reimbursement for out-of-pocket expenses they paid to the site of care. Irrespective of who will receive the co-pay payment, the site of care must first file a claim for MYOBLOC and the related injection-administration expenses with the patient’s private insurance carrier(s). An Explanation of Benefits that shows payment for MYOBLOC and the related injection expenses is required with supporting evidence to establish out-of-pocket expenses before any such reimbursement is authorized by the MYOBLOC Co-pay Program [See Reference 1 for Michigan, Rhode Island, and Minnesota residents].
-
Upon approval into the program, eligible costs for the patient’s MYOBLOC injections may be submitted for payment. The program administrator will verify that the costs are eligible for payment. Payment for eligible costs will be issued to the site of care via a virtual credit card number within 2 business days of the receipt of information validating eligible out-of-pocket expenses. If the site of care requires a check reimbursement, that check will be issued and mailed within 3-4 weeks. Any pharmacy using the Co-pay Program may use the patient’s card ID, Rx and BIN to process and receive payment on claims.
-
1. This offer is valid for commercially insured patients only and is good for use only with a MYOBLOC prescription at the time the prescription is filled or after the product is administered to the patient. 2. Depending on insurance coverage, eligible insured patients may pay no more than zero dollars ($0) for MYOBLOC and the administrative services associated with MYOBLOC, up to a maximum savings limit of four thousand dollars ($4,000) per year. Patient out-of-pocket expense may vary. 3. This offer is not valid for patients enrolled in Medicare, Medicaid, or other federal or state healthcare programs, or private indemnity or HMO insurance plans that reimburse you for the entire cost of your prescription drugs. Patients may not use this Program if they are Medicare-eligible and enrolled in an employer-sponsored health plan or medical or prescription drug benefit program for retirees. 4. The offer is valid for one (1) year. 5. Supernus reserves the right to rescind, revoke, or amend this offer without notice. 6. Offer good only in the USA, including Puerto Rico, at participating pharmacies or Healthcare Providers. 7. Void if prohibited by law, taxed, or restricted. 8. Residents of Michigan, Rhode Island, and Minnesota are not eligible for assistance with payment for injection or injection guidance-related costs, but may receive assistance with MYOBLOC. 9. This Program is not transferable. The selling, purchasing, trading, or counterfeiting of this Program is prohibited by law. 10. This Program is not insurance. 11. By redeeming this assistance, you represent that, to the best of your knowledge, the patient is eligible to participate in the Program and that you understand and agree to comply with the terms and conditions of this offer.
Patients are free, at any time, to switch Healthcare Providers, practitioners, pharmacies, commercial insurers, or suppliers without affecting continued eligibility for assistance. If patients begin receiving benefits from a government program, they would become ineligible for the Co-pay Assistance Program for MYOBLOC.
Submitting an application for assistance does not guarantee funding will be available. If financial assistance is awarded, it will be provided on an annual basis. Applicants must reapply for assistance each year. Funding in any subsequent year(s) or time frames is not guaranteed. The Co-pay Assistance Program for MYOBLOC may be modified or discontinued at any time. NOTE: Reimbursement services are available only for those patients being treated with MYOBLOC for a therapeutic condition for which there is a reasonable expectation of reimbursement from a third-party payer. Physicians are responsible for identifying the clinical indication and documenting medical necessity for use of MYOBLOC. Questions regarding the clinical use of MYOBLOC should be directed to 1-888-461-2255, Option 2.
Patient Assistance Program
-
MYOBLOC is available at no charge to eligible patients who are approved for the Patient Assistance Program.
Eligibility Criteria:
- Patients for whom MYOBLOC is prescribed by a licensed physician to treat cervical dystonia or chronic sialorrhea
- Patients who have no health insurance benefits for MYOBLOC
- Insured - No benefits
- Uninsured
- Patient cannot otherwise afford MYOBLOC therapy
- Patient is a legal United States resident
- Patient is injected with MYOBLOC (J-Code J0587) accompanied with ICD-10 code G24.3 for cervical dystonia or ICD-10 code K11.7 for chronic sialorrhea
-
For patients who are approved for the Patient Assistance Program:
- MYOBLOC is provided at no charge
- MYOBLOC is shipped directly to the healthcare provider marked for patient use
- Qualified patients are eligible to receive MYOBLOC injections for two (2) dates of services provided that they do not subsequently gain health insurance benefits
- Patient may re-apply for additional dates of service
- Patient is responsible for any charges related to Healthcare Provider
Solstice Neurosciences, LLC (“Solstice Neurosciences”), a wholly-owned subsidiary of Supernus Pharmaceuticals, sets the criteria for the Patient Assistance Program. Acceptance into the program at any time is not a guarantee that patients are entitled to receive assistance indefinitely.
Solstice Neurosciences reserves the right at any time, and without notice, to modify or discontinue any or all of the aspects of the Program; or to terminate assistance under the Program at any time.
Take the next step with MYOBLOC
-

Order MYOBLOC
Order MYOBLOCOrder by phone or email and we'll help you get the treatment your patients need.
-

Get Live Support
Request A SpecialistGet in touch with a Reimbursement Specialist who can help you navigate patient support services, insurance benefits, and more.
-

Request Samples
Request SamplesEasy online sample ordering to help you and your patients get started with MYOBLOC.
